Abstract
Background
Venous thromboembolism (VTE) is a leading cause of mortality in patients with cancer and is associated with significant morbidity and healthcare expenditure. The risk of VTE is also increased following the insertion of a central venous catheter (CVC) for chemotherapy deliverance and supportive care. The risks and benefits of primary thromboprophylaxis in patients with cancer and newly inserted CVC are unclear.
Objective
We sought to assess the rates of VTE and major bleeding complications to determine the safety and efficacy of primary thromboprophylaxis in adult patients with cancer and a CVC.
Methods
A systematic search of MEDLINE, EMBASE, and all EBM was conducted. Randomized controlled trials (RCTs) of adult patients with cancer and a CVC receiving primary thromboprophylaxis or observation/placebo were included. The primary efficacy and safety outcomes were total VTE and major bleeding episodes, respectively.
Results
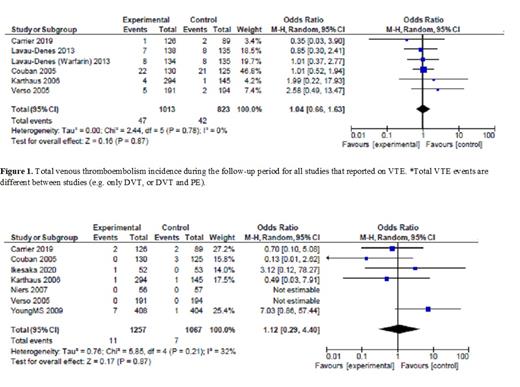
A total of 9 RCTs (3155 patients) were included in the analysis. The total rates of VTE were significantly lower in patients receiving primary thromboprophylaxis compared to those not receiving primary prevention (7.6% vs. 13%; Odds Ratio (OR) 0.51, 95% CI 0.32 to 0.82, p < 0.01, I² = 52%) (Figure 1). The rate of major bleeding complication was not increased in patients receiving thromboprophylaxis (0.9% vs. 0.7%; OR 1.12, 95% CI 0.29 to 4.40, p = 0.87, I² = 32%) (Figure 2).
Conclusions
Primary thromboprophylaxis significantly reduced the risk of VTE without increasing the risk of major bleeding complications in patients with cancer and CVC. Future studies are needed to confirm these findings.
Wang: Servier: Membership on an entity's Board of Directors or advisory committees; Leo Pharma: Research Funding. Ikesaka: LEO Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees. Wells: Bristol-Myers Squibb: Honoraria; Pfizer: Honoraria; Bayer: Honoraria; BMS/Pfizer: Research Funding; Servier: Honoraria. Carrier: Servier: Honoraria; Boehringer Ingelheim: Honoraria; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Aspen: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; LEO Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi Aventis: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal